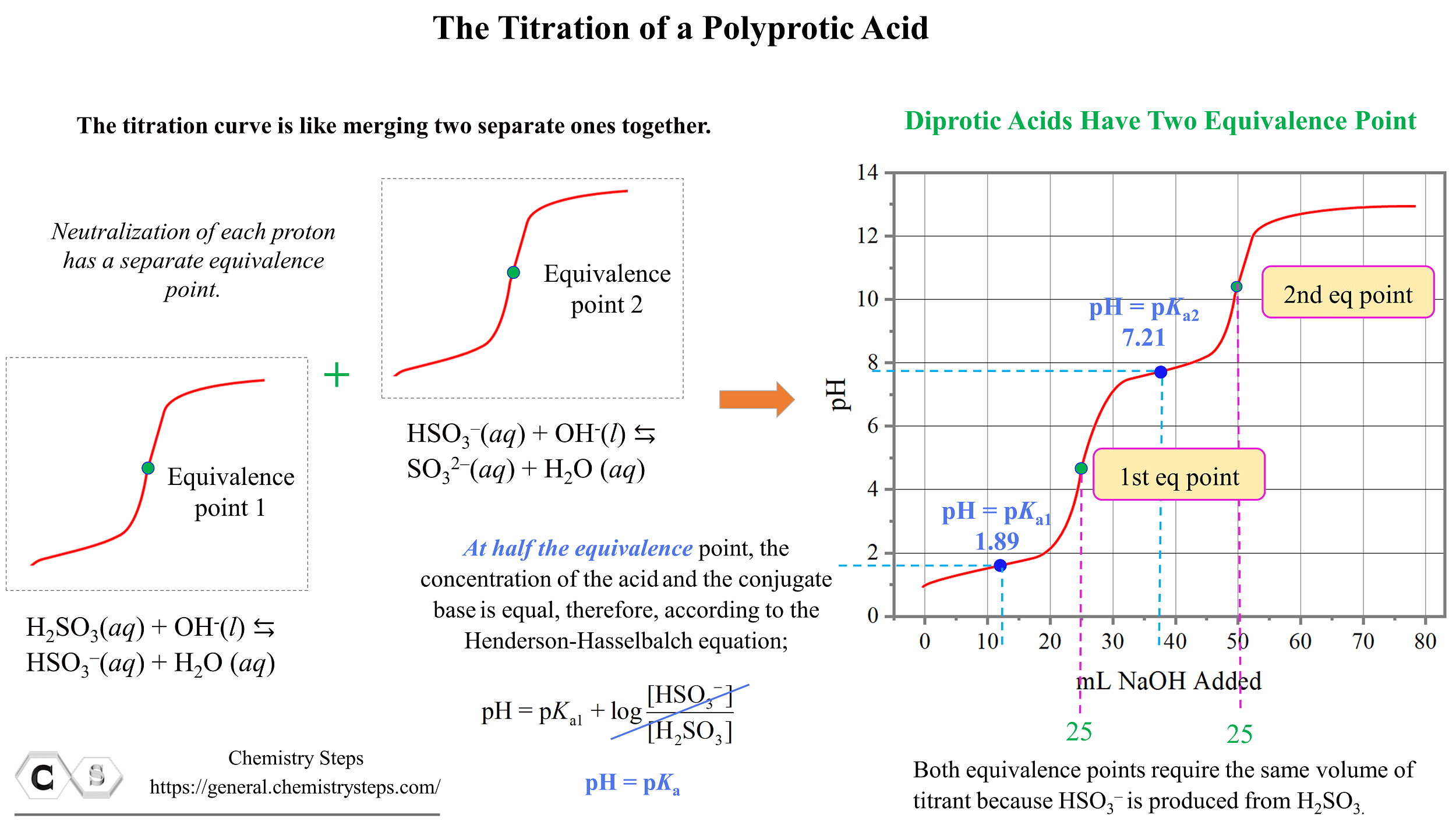
Titration Ph Formula . calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Learn the titration graph and titration equation. What is the equivalence point of a titration. When you carry out a. The reaction can be represented as: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. What are titrant and analyte. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh.
from general.chemistrysteps.com
When you carry out a. What are titrant and analyte. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. The reaction can be represented as: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. What is the equivalence point of a titration. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Learn the titration graph and titration equation.
Titration of a Polyprotic Acids Chemistry Steps
Titration Ph Formula 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. The reaction can be represented as: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When you carry out a. What are titrant and analyte. Learn the titration graph and titration equation. What is the equivalence point of a titration. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base:
From slideplayer.com
Acid Base Titration Curves & Indicators ppt download Titration Ph Formula 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. When you carry out a. What is the equivalence point of a titration. What are titrant and analyte. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Learn the. Titration Ph Formula.
From mungfali.com
Acid Base Titration Calculation Titration Ph Formula When you carry out a. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Learn the titration graph and titration equation. The reaction can be represented as: What is the equivalence point of a titration. What are titrant and analyte. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate. Titration Ph Formula.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry Titration Ph Formula When you carry out a. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. What are titrant and analyte. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The reaction can be represented as: What is the equivalence. Titration Ph Formula.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download ID2145668 Titration Ph Formula 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. What is the equivalence point of a titration. What are titrant and analyte. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Consider the titration of 25.00 ml of. Titration Ph Formula.
From www.youtube.com
pH changes in an acidbase titration YouTube Titration Ph Formula When you carry out a. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. At the equivalence point in a. Titration Ph Formula.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Ph Formula Learn the titration graph and titration equation. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. What is the equivalence point of a titration. The reaction can be represented as: What are titrant and analyte. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph for the strong. Titration Ph Formula.
From mungfali.com
Acid Base Titration Calculation Titration Ph Formula What is the equivalence point of a titration. Learn the titration graph and titration equation. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: At the equivalence point in a neutralization, the moles of acid are equal to. Titration Ph Formula.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Ph Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. What is the equivalence point of a titration. What are titrant and analyte. Learn the titration graph and titration equation. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. The reaction can be represented as: When you carry out a. Consider the. Titration Ph Formula.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Titration Ph Formula What is the equivalence point of a titration. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The reaction can. Titration Ph Formula.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Ph Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. What is the equivalence point of a titration. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. Learn the titration graph and titration equation. What are titrant and analyte. 0.00 ml, 15.0 ml,. Titration Ph Formula.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with potassium permanganate is 2 Titration Ph Formula What are titrant and analyte. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph for the strong acid/strong base titration. Titration Ph Formula.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax General Chemistry Titration Ph Formula When you carry out a. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Learn the titration graph and titration equation. What is the equivalence point of a titration. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph. Titration Ph Formula.
From www.youtube.com
Calculating pH During Titration Reactions YouTube Titration Ph Formula What are titrant and analyte. The reaction can be represented as: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. What is the equivalence point of a titration. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh. Titration Ph Formula.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Ph Formula calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The reaction can be represented as: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When you carry out a.. Titration Ph Formula.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Titration Ph Formula calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: What are titrant and analyte. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. At the equivalence point in a neutralization, the moles of acid are equal to the. Titration Ph Formula.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Ph Formula The reaction can be represented as: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When you carry out a. Learn the titration graph and titration equation. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with. Titration Ph Formula.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Ph Formula At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. What are titrant and analyte. What is the equivalence point of a titration. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate. Titration Ph Formula.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Ph Formula Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. What is the equivalence point of a titration. What are titrant and analyte. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate the ph for the strong acid/strong base titration between. Titration Ph Formula.